Taking the lab out of testing in the COVID-19 pandemic
Point-of-care testing (POCT) has been heralded as the potential solution to numerous healthcare problems for some time. These miniaturised lab-on-chip devices have the capacity to decentralise testing, accelerate the turnaround of results, streamline care pathways and – eventually – save money.
Some of the biggest healthcare challenges we face could be tackled by the use of POCT and home testing. The approach is particularly attractive for those who want or need a remote diagnosis, which currently is pretty much all of us….
Like many technologies with a remote capacity, the COVID-19 pandemic has sped up the development of point-of-care and home testing devices, prompting some innovators to pivot their technology for COVID-19 testing whilst others are accelerating development of their technology for use with a range of health conditions.
It has been anticipated that the global market for these devices will grow substantially in coming years.
But what does this mean for patients and those working in the NHS? How can this growth improve healthcare services during the pandemic and beyond? And how can we make sure that we get the right test for the job?
Developments and advances
Even before COVID-19 there have been a number of technological advances and systemic changes to help the development of point-of-care devices.
The technology of POCT has made huge advances and, as accuracy and speed of results improves, the technologies are better meeting patient needs by removing clinical uncertainty, providing faster diagnosis and informing decision-making.
Quidel’s point of care test for pre-eclampsia provides a nice example of this functionality. Pre-eclampsia occurs in around four per cent of all pregnancies and, in extreme cases, it can lead to foetal or maternal death.
Because of its potentially fatal outcome clinical teams have a low threshold to admit pregnant women with suspected pre-eclampsia, placing significant economic and capacity burdens on maternity systems. Up until to now there has been no definitive way to accurately diagnose who is not at risk, meaning women are routinely admitted ‘just in case’ although most do not have the condition.
Pre-eclampsia occurs as a result of a poorly functioning placenta and the Quidel Triage PLGF test assesses this by measuring the levels of placental growth factor (PLGF) and applying a clinical algorithm to place the patient in one of three risk groups.
Part of NHS England’s Innovation and Technology Payment Programme, Quidel’s PLGF test has been shown to be a more accurate predictor of pregnancy outcome than all standard tests and has helped clinicians and midwives make quicker, more effective clinical decisions about whether or not to admit an expectant mother to hospital. The predicted cost savings are £635 per woman with suspected pre-eclampsia and the savings from providing peace of mind to expectant mothers and their families are invaluable.
Quidel Triage PLGF test can be utilised in both laboratory and point of care settings. This provides flexibility, to best meet the demands of Trusts incorporating the test into clinical practice. Currently the test is used in Whittington Hospital, in a laboratory based setting.

Point-of-care tests for COVID-19
WHO has identified POCT as the first of eight research priorities in the COVID-19 outbreak. This approach has clear advantages in managing a pandemic in terms of rapid testing to inform decisions around treatment and, in hospitals, quicker testing can improve patient flow and reduce the likelihood of transmission within the hospital setting.
A large number of POCT devices have been developed and adapted to detect COVID-19 infection. Imperial College London spin-out DNA-Nudge is one example which has adapted its genetic testing service that informs consumers’ dietary decisions into a diagnostic test for the virus.
The 90 minute COVID Nudge RT-PCR platform has been trialled in a number of settings across London and Oxford showing sensitivity of 94% compared to lab PCR tests. It can detect the presence of human RNA as well as viral RNA to eliminate ‘false negatives’ providing a specificity of 100%. And in terms of clinical impact it has shown a positive effect on infection control measures, patient flow and patient enrolment in clinical trials.
The UK Government is currently rolling out a number of POC lateral-flow solutions which are less accurate than PCR tests but have a very fast turnaround and could potentially be self-administered. Evaluations suggest that lateral flow tests should be used to test asymptomatic people, as they offer the advantage of reducing risk and increasing capacity but there is still debate as to the value of the tests considering their accuracy.
Another POC approach is to use tests to differentiate between viral and and bacterial causes of respiratory infection as a means to triage potential COVID-19 patients. FebriDx provides a rapid triaging solution by distinguishing between patients with COVID-19 symptoms that do and do not have a respiratory viral infection. It enables those without a viral infection to be directed to non-COVID19 areas, reducing the risk of infection and improving patient flow. A triage algorithm using a combination of FebriDx point of care assay and clinical parameters is being trialled for medical admissions of possible COVID-19 patients at Northwick Park Hospital. The evaluation has shown it is useful to rule out COVID-19 infection and manage medical admissions from the emergency department.

This diversity in POCT approaches for COVID-19 infection demonstrate that the choice of technology depends on who will be tested and the environment in which it is deployed. It is by no means a ‘one test fits all’ situation.
The need for POC and remote testing in a pandemic
Current measures around social distancing, quarantine and shielding are creating a more pressing demand for POCT devices for a range of health needs.
One of these groups are the over 65s and particularly those with acute frailty syndrome. Acute frailty symptoms such as falls, confusion, dizziness can be benign but they can also mask more serious underlying conditions so it’s crucial to ascertain various health measurements when they are first seen to guide patient management.
The Abbott i-STAT Alinity point of care solution can perform a range of cardiac, haematology, endocrinology and blood gas tests at the point of care, providing results in approximate two minutes. The technology has been trialled by ambulance services to aid decision making for patients over 65 with acute frailty syndrome and showed an improvement in clinical confidence and earlier disease management.
It is also in use by Physician Response Unit which provides a physician-paramedic team to respond to emergency calls to enable fewer hospitalisations and by community respiratory teams across London to assess, treat and monitor patients and help them self-manage their respiratory disease.
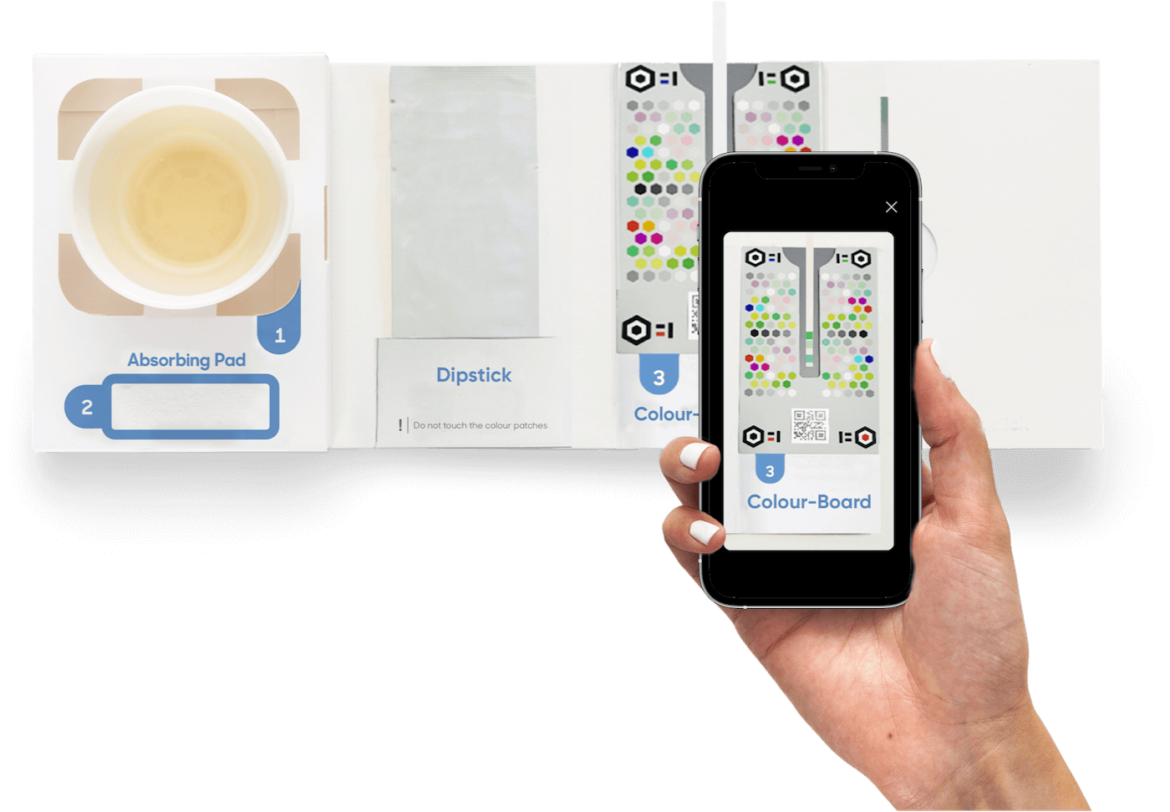
There are also remote ‘at home’ tests that can support health services and patients at this time. Digital at home kidney health testing from Healthy.io is helping relieve stretched capacity in primary care and allowing vulnerable patients at increased risk of COVID-19 to stay at home by enabling them to complete their annual urine test without visiting the GP’s practice. The CE marked home testing kit and app is helping increase the number of people with diabetes who take an annual urinal albumin screening test, identifying more cases of chronic kidney disease earlier, when they are still more treatable. Results are integrated directly into the GP’s record. Supported by CareCity and UCLPartners this is currently in use in East London and many other NHS sites UK wide.
In partnership with NHSx, the Accelerated Access Collaborative and the National Institute for Health Research, Healthy.io has received funding to help shift kidney health testing from the clinic to the home across the UK. The project is aiming to release primary care capacity by allowing people at increased risk of Chronic Kidney Disease (CKD) to complete their annual urine test at home.
There are clear advantages to POCT and remote testing, especially in the time of a pandemic. Alongside providing fast, accurate diagnoses it can avoid logistical errors such as loss of sampling or mislabelling. The approach has the potential to ease decision making, lead to a more streamlined pathway and, in many cases, enable patients to take more control over their own care. Now more than ever it’s important for patients and clinicians to know quickly not only when a test is positive but when it is a negative to avoid unnecessary hospitalisation and patient concern.
If any of these technological solutions are of interest or if you have any innovations you would like to share in this area please do get in contact with the Commercial & Innovation team at UCLPartners.